Box and Arrow Orbital Configurations using
Pauli Exclusion Principle and Hund's Rule
There is yet another way to writing electron configurations. It is called the "Box and Arrow" (or circle and X) orbital configuration.
Sublevels can be broken down into regions called "orbitals". An orbital is defined as the most probable location for finding an electron. Each orbital holds 2 electrons.
This sublevel configuration can be broken down into orbitals (boxes).
1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 .
Video below on how the sublevels build.
There are a few rules for the box and arrow configurations.
Aufbau Principle - electrons fill orbitals starting at the lowest available energy state before filling higher states (1s before 2s).
Pauli Exclusion Principle
An orbital can hold 0, 1, or 2 electrons only, and if there are two electrons in the orbital, they must have opposite (paired) spins.
When we draw electrons, we use up and down arrows. So, if an electron is paired up in a box, one arrow is up and the second must be down.
(Therefore, no two electrons in the same atom can have the same set of four Quantum Numbers . )
incorrect; electrons must spin in opposite directions
correct; the electrons have opposite spins
When filling sublevels other than s, electrons are placed in individual orbitals before they are paired up.
Electrons fill like people do on a bus. You would never sit right next to someone you did not know if there are free seats available, unless of course all the seats are taken then you must pair up.
So when working with the p sublevel, electrons fill like this. up, up, up. down, down, down. take a look
US Search Desktop

We appreciate your feedback on how to improve Yahoo Search. This forum is for you to make product suggestions and provide thoughtful feedback. We’re always trying to improve our products and we can use the most popular feedback to make a positive change!
If you need assistance of any kind, please visit our community support forum or find self-paced help on our help site. This forum is not monitored for any support-related issues.
The Yahoo product feedback forum now requires a valid Yahoo ID and password to participate.
You are now required to sign-in using your Yahoo email account in order to provide us with feedback and to submit votes and comments to existing ideas. If you do not have a Yahoo ID or the password to your Yahoo ID, please sign-up for a new account.
If you have a valid Yahoo ID and password, follow these steps if you would like to remove your posts, comments, votes, and/or profile from the Yahoo product feedback forum.
- Vote for an existing idea ( )
- or
- Post a new idea…
- Hot ideas
- Top ideas
- New ideas
- Category
- Status
- My feedback
Put on the computer what the public asks for, not everything about the US.
I asked for a total medal count. I have been looking for 20 minutes and still cannot find it.
I also asked for a specific medal count for a specific country and got a history of when they first started to compete in the Olympics. I asked for a medal count for 2018, as of today,
not a history of that country.
You don't even accept what I have asked.
- Don't see your idea?
- Post a new idea…
US Search Desktop
- Post a new idea…
- All ideas
- My feedback
- I have a problem 24
- I have a suggestion 20
- Other 3
- What I dislike 29
Feedback and Knowledge Base
Give feedback
- Deutschland Finanzen Mobile DF iOS 1 idea
- España Finanzas Mobile DF iOS 7 ideas
- Accounts Dashboard 33 ideas
- Ad feedback 3 ideas
- Answers TH 31 ideas
- Answers TH 0 ideas
- Answers UV Forum (test version) 10 ideas
- Australia Celebrity 0 ideas
- Australia Finance Mobile Android 0 ideas
- Australia Style 0 ideas
- Australia Yahoo Tech 0 ideas
- Autos Pulse 2 ideas
- Aviate 1,513 ideas
- Canada Finance 1,099 ideas
- Canada Finance Mobile Android 0 ideas
- Canada Finance Mobile DF iOS 3 ideas
- Canada Finance Mobile iOS 469 ideas
- Canada Homepage 5,130 ideas
- Canada Movies 14 ideas
- Canada News 873 ideas
- Canada Safely 10 ideas
- Canada Screen 128 ideas
- Canada Weather 94 ideas
- Canada Yahoo Beauty 0 ideas
- Canada Yahoo Celebrity 10 ideas
- Canada Yahoo Finance 0 ideas
- Canada Yahoo Movies 10 ideas
- Canada Yahoo News 0 ideas
- Canada Yahoo Style 21 ideas
- College Football Pick'em 112 ideas
- Connected TV 362 ideas
- Corp Mail Test 1 1,313 ideas
- Corp Mail Testing 1,256 ideas
- Cricket 24 ideas
- Daily Fantasy 89 ideas
- Developer Network 1 idea
- Double Down 86 ideas
- Fantasy Baseball 455 ideas
- Fantasy Basketball 402 ideas
- Fantasy Football 707 ideas
- Fantasy Hockey 354 ideas
- Fantasy Live Scoring on Matchup and Standings 811 ideas
- Fantasy Ratings and Levels 7 ideas
- Fantasy Sports Android Apps 1,367 ideas
- Fantasy Sports iOS Apps 2,127 ideas
- Finance 1,248 ideas
- Finance - CA 495 ideas
- Finance - US 9 ideas
- Finance ChartIQ 443 ideas
- Finance Mobile Web 403 ideas
- Finance Portfolios 810 ideas
- Finance Stock Screener 35 ideas
- Finance Tablet 44 ideas
- Flickr - Profile 290 ideas
- Flickr Android 60 ideas
- Flickr for Apple TV 27 ideas
- Flickr Groups 13 ideas
- Flickr Internal 0 ideas
- Flickr iOS Dogfooding 0 ideas
- Flickr iPad 152 ideas
- Flickr iPhone 370 ideas
- Flickr New Photo Page 8,030 ideas
- Flickr Search 0 ideas
- Food Magazines 0 ideas
- Games 3,147 ideas
- Global Maps 1,024 ideas
- GS Mobile Web 42 ideas
- Health Pulse 3 ideas
- Home Page (Android) 1,689 ideas
- Home Page (iOS) 3,809 ideas
- Hong Kong Homepage 0 ideas
- India Celebrity 43 ideas
- India Finance 493 ideas
- India Homepage 1,872 ideas
- India Lifestyle 173 ideas
- India Movies 84 ideas
- India News 334 ideas
- India Partner Portal Tata 0 ideas
- India Partner Portal Tikona 0 ideas
- India Safely 15 ideas
- India Screen 165 ideas
- India Weather 30 ideas
- India Yahoo Beauty 0 ideas
- India Yahoo Celebrity 4 ideas
- India Yahoo Finance 0 ideas
- India Yahoo Movies 16 ideas
- India Yahoo News 0 ideas
- India Yahoo Style 14 ideas
- Indonesia Celebrity 38 ideas
- Indonesia Homepage 1,165 ideas
- Indonesia News 170 ideas
- Indonesia Safely 29 ideas
- Indonesia She 34 ideas
- Ireland Homepage 90 ideas
- Jordan Maktoob Homepage 419 ideas
- Mail Ad Feedback 10 ideas
- Maktoob الطقس مكتوب 5 ideas
- Maktoob Celebrity 1 idea
- Maktoob Entertainment 10 ideas
- Maktoob Lifestyle 0 ideas
- Maktoob Movies 2 ideas
- Maktoob News 182 ideas
- Maktoob Screen 15 ideas
- Maktoob Style 1 idea
- Maktoob ألعاب مكتوب 0 ideas
- Maktoob شاشة مكتوب 28 ideas
- Malaysia Homepage 17 ideas
- Malaysia News 58 ideas
- Malaysia Safely 7 ideas
- Malaysia Video 0 ideas
- Malaysia Weather 1 idea
- Merchant Solutions 1 idea
- My Yahoo 31,967 ideas
- My Yahoo - back up 1 idea
- My Yahoo - US 9,176 ideas
- My Yahoo archive 314 ideas
- New Mail 11,359 ideas
- New Mail* 3,165 ideas
- New Zealand Business & Finance 132 ideas
- New Zealand Homepage 1,039 ideas
- New Zealand Safely 3 ideas
- New Zealand Screen 0 ideas
- PH ANC News 21 ideas
- Philippines Celebrity 214 ideas
- Philippines Homepage 9 ideas
- Philippines News 123 ideas
- Philippines Safely 12 ideas
- Philippines Video 0 ideas
- Philippines Weather 3 ideas
- Pick N Roll 19 ideas
- Postmaster 43 ideas
- Pro Football Pick'em 103 ideas
- Retail Pulse 0 ideas
- Rivals 11 ideas
- Safely 165 ideas
- Screen for iOS 0 ideas
- Search Extensions 98 ideas
- Search Product Downloads 89 ideas
- Security 497 ideas
- Sign-In Experience 79 ideas
- Singapore Entertainment 20 ideas
- Singapore Finance 230 ideas
- Singapore Homepage 1,052 ideas
- Singapore News 214 ideas
- Singapore Safely 11 ideas
- Singapore Screen 19 ideas
- Singapore Weather 4 ideas
- Singapore Yahoo Beauty 0 ideas
- Singapore Yahoo Celebrity 4 ideas
- Singapore Yahoo Finance 0 ideas
- Singapore Yahoo Movies 0 ideas
- Singapore Yahoo News 0 ideas
- Singapore Yahoo Style 4 ideas
- South Africa Celebrity 8 ideas
- South Africa Homepage 374 ideas
- South Africa News 23 ideas
- Sports Android 1,534 ideas
- Sports CA 35 ideas
- Sports iOS 1,026 ideas
- Sports Redesign 3,206 ideas
- SportsReel 6 ideas
- StatTracker Beta 581 ideas
- Survival Football 81 ideas
- Taiwan Yahoo 名人娛樂 0 ideas
- Taiwan Yahoo 運動 0 ideas
- Test 0 ideas
- Thailand Safely 2 ideas
- Toolbar Mail App 216 ideas
- Toolbar Weather App 72 ideas
- Tourney Pick'em 44 ideas
- UK & Ireland Finance 1,077 ideas
- UK & Ireland Games 19 ideas
- UK & Ireland Homepage 455 ideas
- UK & Ireland News 0 ideas
- UK & Ireland News Internal bucket 0 ideas
- UK & Ireland News Lego 378 ideas
- UK & Ireland Safely 38 ideas
- UK & Ireland TV 21 ideas
- UK & Ireland Video 187 ideas
- UK & Ireland Weather 100 ideas
- UK Answers 1 idea
- UK Daily Fantasy 1 idea
- UK Finance Mobile Android 12 ideas
- UK Finance Mobile DF iOS 2 ideas
- UK Finance Mobile iOS 310 ideas
- UK Yahoo Movies 23 ideas
- US Answers 8,999 ideas
- US Answers Mobile Web 2,156 ideas
- US Autos GS 442 ideas
- US Celebrity GS 661 ideas
- US Comments 350 ideas
- US Finance Mobile Android 44 ideas
- US Finance Mobile iOS 582 ideas
- US Flickr 267 ideas
- US Groups 4,225 ideas
- US Homepage B1 68 ideas
- US Homepage B2 33 ideas
- US Homepage B3 50 ideas
- US Homepage B4 33 ideas
- US Homepage B5 0 ideas
- US Homepage M 7,021 ideas
- US Homepage YDC 43 ideas
- US Homes GS 203 ideas
- US Live Web Insights 24 ideas
- US Mail 193 ideas
- US Mail 12,398 ideas
- US Maps 3,491 ideas
- US Membership Desktop 8,189 ideas
- US Membership Mobile 91 ideas
- US Movies GS 424 ideas
- US Music GS 195 ideas
- US News 6,057 ideas
- US Search App Android 2 ideas
- US Search App iOS 13 ideas
- US Search Chrome Extension 780 ideas
- US Search Chrome Extension v2 2,197 ideas
- US Search Desktop 1 idea
- US Search Desktop Bucket A 7 ideas
- US Search Desktop Bucket B 8 ideas
- US Search KG 14 ideas
- US Search Local Listings 20,797 ideas
- US Search Mobile Web 1 idea
- US Search Mozilla 0 ideas
- US Search Stock Quotes 11 ideas
- US Search Tablet Web 1 idea
- US Shine GS 1 idea
- US Toolbar 5,548 ideas
- US Travel GS 207 ideas
- US TV GS 367 ideas
- US Weather 2,322 ideas
- US Weather Bucket 0 ideas
- US Weather Mobile 13 ideas
- US Weather Mobile Android 2 ideas
- Video Guide Android 150 ideas
- Video Guide iOS 207 ideas
- Video Guide Testing 15 ideas
- Web Hosting 4 ideas
- Whitelist Yahoo Mail 0 ideas
- Yahoo Accessibility 359 ideas
- Yahoo Autos 71 ideas
- Yahoo Beauty 102 ideas
- Yahoo Celebrity 0 ideas
- Yahoo Celebrity Canada 0 ideas
- Yahoo Decor 0 ideas
- Yahoo Entertainment 357 ideas
- Yahoo Esports 50 ideas
- Yahoo Feedback 0 ideas
- Yahoo Finance Feedback Forum 1 idea
- Yahoo Finance IN Mobile Android 0 ideas
- Yahoo Finance SG Mobile Android 1 idea
- Yahoo FinanceReel 4 ideas
- Yahoo Food 118 ideas
- Yahoo Gemini 2 ideas
- Yahoo Health 90 ideas
- Yahoo Help 340 ideas
- Yahoo Home 240 ideas
- Yahoo Home* 28 ideas
- Yahoo Lifestyle 168 ideas
- Yahoo Live 0 ideas
- Yahoo Mail 2,348 ideas
- Yahoo Mail Android App 415 ideas
- Yahoo Mail Basic 643 ideas
- Yahoo Mail iOS App 55 ideas
- Yahoo Mail Mobile Web 1 idea
- Yahoo Makers 51 ideas
- Yahoo Messenger 93 ideas
- Yahoo Mobile Developer Suite 61 ideas
- Yahoo Mobile for Phone 15 ideas
- Yahoo Mobile for Tablet 0 ideas
- Yahoo Music 78 ideas
- Yahoo News Digest Android 870 ideas
- Yahoo News Digest iPad 0 ideas
- Yahoo News Digest iPhone 1,531 ideas
- Yahoo Newsroom Android App 59 ideas
- Yahoo Newsroom iOS App 34 ideas
- Yahoo Parenting 63 ideas
- Yahoo Politics 118 ideas
- Yahoo Publishing 13 ideas
- Yahoo Real Estate 2 ideas
- Yahoo Tech 461 ideas
- Yahoo Travel 143 ideas
- Yahoo TV 103 ideas
- Yahoo View 217 ideas
- Yahoo Weather Android 2,142 ideas
- Yahoo Weather iOS 22,810 ideas
- Yahoo! 7 Food App (iOS) 0 ideas
- Yahoo! 7 Homepage Archive 57 ideas
- Yahoo! 7 News (iOS) 23 ideas
- Yahoo! 7 Screen 0 ideas
- Yahoo! 7 TV FANGO App (Android) 1 idea
- Yahoo! 7 TV FANGO App (iOS) 1 idea
- Yahoo! 7 TV Guide App (Android) 0 ideas
- Yahoo! 7 TV Guide App (iOS) 1,249 ideas
- Yahoo! 7 TV Plus7 App (iOS) 0 ideas
- Yahoo! Concept Test Feedback Center 174 ideas
- Yahoo! Contributor Network 1 idea
- Yahoo! Transliteration 29 ideas
- YAHOO!7 Finance 553 ideas
- Yahoo!7 Games 9 ideas
- Yahoo!7 Safely 19 ideas
- Yahoo7 Finance Mobile DF iOS 12 ideas
- Yahoo7 Finance Mobile iOS 217 ideas
- Yahoo7 Homepage 2,549 ideas
Your password has been reset
We have made changes to increase our security and have reset your password.
We've just sent you an email to . Click the link to create a password, then come back here and sign in.
Hund's Rules
The Aufbau section discussed how that electrons fill the lowest energy orbitals first, and then move up to higher energy orbitals only after the lower energy orbitals are full. However, there a problem with this rule. Certainly, 1s orbitals should be filled before 2s orbitals, because the 1s orbitals have a lower value of n, and thus a lower energy. What about the three different 2p orbitals? In what order should they be filled? The answer to this question involves Hund's rule.
Hund's rule states that:
- Every orbital in a sublevel is singly occupied before any orbital is doubly occupied.
- All of the electrons in singly occupied orbitals have the same spin (to maximize total spin).
When assigning electrons to orbitals, an electron first seeks to fill all the orbitals with similar energy (also referred to as degenerate orbitals) before pairing with another electron in a half-filled orbital. Atoms at ground states tend to have as many unpaired electrons as possible. In visualizing this process, consider how electrons exhibit the same behavior as the same poles on a magnet would if they came into contact; as the negatively charged electrons fill orbitals, they first try to get as far as possible from each other before having to pair up.
Example \(\PageIndex<1>\): Nitrogen Atoms
Consider the correct electron configuration of the nitrogen (Z = 7) atom: 1s 2 2s 2 2p 3
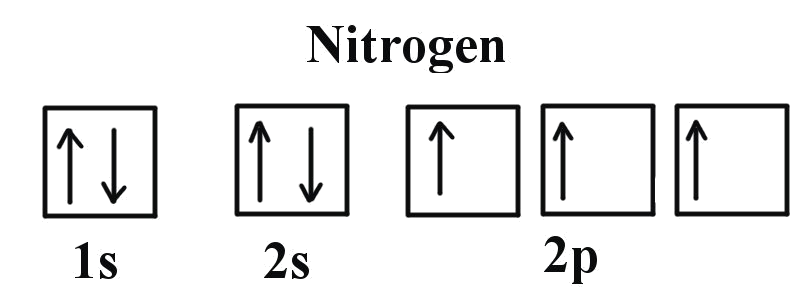
The p orbitals are half-filled; there are three electrons and three p orbitals. This is because the three electrons in the 2p subshell will fill all the empty orbitals first before pairing with electrons in them.
Keep in mind that elemental nitrogen is found in nature typically as dinitrogen, N2, which requires molecular orbitals instead of atomic orbitals as demonstrated above.
Example \(\PageIndex<2>\): Oxygen Atoms
Next, consider oxygen (Z = 8) atom, the element after nitrogen in the same period; its electron configuration is: 1s 2 2s 2 2p 4

Oxygen has one more electron than nitrogen; as the orbitals are all half-filled, the new electron must pair up. Keep in mind that elemental oxygen is found in nature typically as dioxygen, \(O_2\), which has molecular orbitals instead of atomic orbitals as demonstrated above.
Hund's Rule Explained
According to the first rule, electrons always enter an empty orbital before they pair up. Electrons are negatively charged and, as a result, they repel each other. Electrons tend to minimize repulsion by occupying their own orbitals, rather than sharing an orbital with another electron. Furthermore, quantum-mechanical calculations have shown that the electrons in singly occupied orbitals are less effectively screened or shielded from the nucleus. Electron shielding is further discussed in the next section.
For the second rule, unpaired electrons in singly occupied orbitals have the same spins. Technically speaking, the first electron in a sublevel could be either "spin-up" or "spin-down." Once the spin of the first electron in a sublevel is chosen, however, the spins of all of the other electrons in that sublevel depend on that first spin. To avoid confusion, scientists typically draw the first electron, and any other unpaired electron, in an orbital as "spin-up."
Example \(\PageIndex<3>\): Carbon and Oxygen
Consider the electron configuration for carbon atoms: 1s 2 2s 2 2p 2 : The two 2s electrons will occupy the same orbital, whereas the two 2p electrons will be in different orbital (and aligned the same direction) in accordance with Hund's rule.
Consider also the electron configuration of oxygen. Oxygen has 8 electrons. The electron configuration can be written as 1s 2 2s 2 2p 4 . To draw the orbital diagram, begin with the following observations: the first two electrons will pair up in the 1s orbital; the next two electrons will pair up in the 2s orbital. That leaves 4 electrons, which must be placed in the 2p orbitals. According to Hund’s rule, all orbitals will be singly occupied before any is doubly occupied. Therefore, two p orbital get one electron and one will have two electrons. Hund's rule also stipulates that all of the unpaired electrons must have the same spin. In keeping with convention, the unpaired electrons are drawn as "spin-up", which gives (Figure 1).
Purpose of Electron Configurations
When atoms come into contact with one another, it is the outermost electrons of these atoms, or valence shell, that will interact first. An atom is least stable (and therefore most reactive) when its valence shell is not full. The valence electrons are largely responsible for an element's chemical behavior. Elements that have the same number of valence electrons often have similar chemical properties.
Electron configurations can also predict stability. An atom is most stable (and therefore unreactive) when all its orbitals are full. The most stable configurations are the ones that have full energy levels. These configurations occur in the noble gases. The noble gases are very stable elements that do not react easily with any other elements. Electron configurations can assist in making predictions about the ways in which certain elements will react, and the chemical compounds or molecules that different elements will form.
Hund's Rules
1. The term with maximum multiplicity lies lowest in energy
Note: Some references, such as Haken & Wolf, use Hund's Rule #1 to apply to the nature of full shells and subshells. Full shells and subshells contribute nothing to the total angular momenta L and S. If you call this Hund'e Rule #1, then the above rules will be bumped up one in number. I don't know which is the more common practice.
Hund's Rule #1
The term with the maximum multiplicity lies lowest in energy.
These sketches are conceptual only. No attempt has been made to do any realistic scaling.
Note that the energies we are discussing here are electric potential energies, so that a negative electron in the vicinity of a positive nucleus will have a negative energy leading to a bound state. Any force between the electrons will tend to counter that, contributing a positive potential energy which makes the electrons less tightly bound, or higher in potential energy.
Hund's Rule #2
For a given multiplicity, the term with the largest value of L lies lowest in energy.
The basis for this rule is essentially that if the electrons are orbiting in the same direction (and so have a large total angular momentum) they meet less often than when they orbit in opposite directions. Hence their repulsion is less on average when L is large.
These influences on the atomic electron energy levels is sometimes called the orbit-orbit interaction. The origin of the energy difference lies with differences in the coulomb repulsive energies between the electrons.
For large L value, some or all of the electrons are orbiting in the same direction. That implies that they can stay a larger distance apart on the average since they could conceivably always be on the opposite side of the nucleus. For low L value, some electrons must orbit in the opposite direction and therefore pass close to each other once per orbit, leading to a smaller average separation of electrons and therefore a higher energy.
Hund's Rule #3
For atoms with less than half-filled shells, the level with the lowest value of J lies lowest in energy.
When the shell is more than half full, the opposite rule holds (highest J lies lowest). The basis for the rule is the spin-orbit coupling. The scalar product S·L is negative if the spin and orbital angular momentum are in opposite directions. Since the coefficient of S·L is positive, lower J is lower in energy.
Exceptions to Hund's Rules
Hund's rules presume L-S coupling and presume that the electrons can be considered to be in a unique configuration. Neither is always true. For heavier elements, the "j-j coupling" scheme often gives better agreement with experiment.
Hund's Rule
Definition - What does Hund's Rule mean?
Hund's rule states that a larger total spin state of an atom sometimes makes the atom more stable.
According to Hund's rule:
- Each orbital in a sublevel is separately occupied before any orbital is doubly occupied.
- All of the electrons in separately occupied orbitals have an equivalent spin (to maximize total spin).
This rule is fairly reliable (with occasional failures) for the determination of the state of a given excited electronic configuration.
Hund's rule is also known as the rule of maximum multiplicity.
Corrosionpedia explains Hund's Rule
According to Hund's rule, electrons are placed into separate orbitals before going into an orbital this is already occupied. This can help predict the properties of atoms, as paired and unmated electrons have distinct properties (specifically with interactions with magnetic fields).
Since electrons are negatively charged, they repel each other. Electrons tend to attenuate repulsion by occupying their own orbitals, instead of sharing an orbital with another electron. For the second rule, unmated electrons in separately occupied orbitals have an equivalent spins. The primary electron in a sublevel could be either "spin-up" or "spin-down."
When atoms come into contact with each other, the outer electrons of those atoms, or valence shell, initially interact. An associate atom is least stable (and therefore most reactive) when its valence shell is not full. The valence electrons are most responsible for an associate element's chemical behavior. Parts that have an equivalent range of valence electrons typically have similar chemical properties.
An associate atom is most stable (and therefore unreactive) once all its orbitals are full of electrons. These configurations are found in the noble gases, which are extremely stable and do not normally react with each other.
Hunds rule?
Trending Now

When electrons are put into orbitals having the same energy, degenerate orbitals, one electron is put into each orbital before putting a second electron into an orbital.
The term with maximum multiplicity (maximum ) has the highest energy level.
For a given multiplicity, the term with the largest value of has the lowest energy in an orbital.
For atoms with less than half-filled shells, the level with the lowest value of lies lowest in energy. Otherwise, if the outermost shell is more than half-filled, the term with highest value of is the one with the lowest energy.
The rules deal in a simple way how the usual energy interactions dictate the ground state term. The rules assume that the repulsion between the outer electrons is very much greater than the spin-orbit interaction which is in turn stronger than any other remaining interactions. This is referred to as the LS coupling regime.
It can be shown that for full orbitals and suborbitals both the residual electrostatic term (repulsion between protons) and the spin-orbit interaction cannot shift all the energy levels together. Thus when determining the ordering of energy levels in general only the inner valence electrons need to be considered.
Due to the Pauli exclusion principle, six electrons cannot share the same set of quantum numbers within the same system. Therefore, there is room for only two electrons in each spatial orbital. One of these electrons must have (for some chosen direction z), , and the other must have . Hund's second rule states that the lowest energy atomic state is the one which maximizes the sum of the values for all of the electrons in the system, maximizing the number of unpaired electrons.
This rule deals again with reducing the repulsion between electrons. It can be understood from the classical picture that if all electrons are orbiting in the same direction (higher orbital angular momentum) they meet less often than if some of them orbit in opposite directions. In that last case the repulsive force increases, which separates electrons. This adds potential energy to them, so their energy level is higher.
This rule considers the energy shifts due to split-orbit coupling. In the case where the spin-orbit coupling is weak compared to the residual electrostatic, where and are still good quantum numbers the splitting is given by:
Hund's rules applied to Si. The up arrows signify electrons with up-spin. The boxes represent different magnetic quantum numbersAs an example, consider the ground state of silicon. The electronic configuration of Si is . Applying the first rule, only the outer electrons need be considered. The possible multiplets are ; of those, and are not allowed because of the exclusion principle. The second rule now states that the triplet state with has the lowest energy. There is no choice of triplets states, so the first rule is not required. [If the state were allowed, then the third rule would come into force and state that it was more favourable than the state.] The triplet consists of three states, . With only two of six possible electrons in the shell, it is less than half-full and thus is the excited state




For atoms with less than half-filled shells, the level with the lowest value of J lies lowest in energy.
When the shell is more than half full, the opposite rule holds (highest J lies lowest). The basis for the rule is the spin-orbit coupling. The scalar product S·L is negative if the spin and orbital angular momentum are in opposite directions. Since the coefficient of S·L is positive, lower J is lower in energy.

Report Abuse
Additional Details
If you believe that your intellectual property has been infringed and would like to file a complaint, please see our Copyright/IP Policy
Report Abuse
Additional Details
If you believe that your intellectual property has been infringed and would like to file a complaint, please see our Copyright/IP Policy
Report Abuse
Additional Details
If you believe that your intellectual property has been infringed and would like to file a complaint, please see our Copyright/IP Policy
US Search Desktop

We appreciate your feedback on how to improve Yahoo Search. This forum is for you to make product suggestions and provide thoughtful feedback. We’re always trying to improve our products and we can use the most popular feedback to make a positive change!
If you need assistance of any kind, please visit our community support forum or find self-paced help on our help site. This forum is not monitored for any support-related issues.
The Yahoo product feedback forum now requires a valid Yahoo ID and password to participate.
You are now required to sign-in using your Yahoo email account in order to provide us with feedback and to submit votes and comments to existing ideas. If you do not have a Yahoo ID or the password to your Yahoo ID, please sign-up for a new account.
If you have a valid Yahoo ID and password, follow these steps if you would like to remove your posts, comments, votes, and/or profile from the Yahoo product feedback forum.
- Vote for an existing idea ( )
- or
- Post a new idea…
- Hot ideas
- Top ideas
- New ideas
- Category
- Status
- My feedback
Put on the computer what the public asks for, not everything about the US.
I asked for a total medal count. I have been looking for 20 minutes and still cannot find it.
I also asked for a specific medal count for a specific country and got a history of when they first started to compete in the Olympics. I asked for a medal count for 2018, as of today,
not a history of that country.
You don't even accept what I have asked.
- Don't see your idea?
- Post a new idea…
US Search Desktop
- Post a new idea…
- All ideas
- My feedback
- I have a problem 24
- I have a suggestion 20
- Other 3
- What I dislike 29
Feedback and Knowledge Base
Give feedback
- Deutschland Finanzen Mobile DF iOS 1 idea
- España Finanzas Mobile DF iOS 7 ideas
- Accounts Dashboard 33 ideas
- Ad feedback 3 ideas
- Answers TH 31 ideas
- Answers TH 0 ideas
- Answers UV Forum (test version) 10 ideas
- Australia Celebrity 0 ideas
- Australia Finance Mobile Android 0 ideas
- Australia Style 0 ideas
- Australia Yahoo Tech 0 ideas
- Autos Pulse 2 ideas
- Aviate 1,513 ideas
- Canada Finance 1,099 ideas
- Canada Finance Mobile Android 0 ideas
- Canada Finance Mobile DF iOS 3 ideas
- Canada Finance Mobile iOS 469 ideas
- Canada Homepage 5,130 ideas
- Canada Movies 14 ideas
- Canada News 873 ideas
- Canada Safely 10 ideas
- Canada Screen 128 ideas
- Canada Weather 94 ideas
- Canada Yahoo Beauty 0 ideas
- Canada Yahoo Celebrity 10 ideas
- Canada Yahoo Finance 0 ideas
- Canada Yahoo Movies 10 ideas
- Canada Yahoo News 0 ideas
- Canada Yahoo Style 21 ideas
- College Football Pick'em 112 ideas
- Connected TV 362 ideas
- Corp Mail Test 1 1,313 ideas
- Corp Mail Testing 1,256 ideas
- Cricket 24 ideas
- Daily Fantasy 89 ideas
- Developer Network 1 idea
- Double Down 86 ideas
- Fantasy Baseball 455 ideas
- Fantasy Basketball 402 ideas
- Fantasy Football 707 ideas
- Fantasy Hockey 354 ideas
- Fantasy Live Scoring on Matchup and Standings 811 ideas
- Fantasy Ratings and Levels 7 ideas
- Fantasy Sports Android Apps 1,367 ideas
- Fantasy Sports iOS Apps 2,127 ideas
- Finance 1,248 ideas
- Finance - CA 495 ideas
- Finance - US 9 ideas
- Finance ChartIQ 443 ideas
- Finance Mobile Web 403 ideas
- Finance Portfolios 810 ideas
- Finance Stock Screener 35 ideas
- Finance Tablet 44 ideas
- Flickr - Profile 290 ideas
- Flickr Android 60 ideas
- Flickr for Apple TV 27 ideas
- Flickr Groups 13 ideas
- Flickr Internal 0 ideas
- Flickr iOS Dogfooding 0 ideas
- Flickr iPad 152 ideas
- Flickr iPhone 370 ideas
- Flickr New Photo Page 8,030 ideas
- Flickr Search 0 ideas
- Food Magazines 0 ideas
- Games 3,147 ideas
- Global Maps 1,024 ideas
- GS Mobile Web 42 ideas
- Health Pulse 3 ideas
- Home Page (Android) 1,689 ideas
- Home Page (iOS) 3,809 ideas
- Hong Kong Homepage 0 ideas
- India Celebrity 43 ideas
- India Finance 493 ideas
- India Homepage 1,872 ideas
- India Lifestyle 173 ideas
- India Movies 84 ideas
- India News 334 ideas
- India Partner Portal Tata 0 ideas
- India Partner Portal Tikona 0 ideas
- India Safely 15 ideas
- India Screen 165 ideas
- India Weather 30 ideas
- India Yahoo Beauty 0 ideas
- India Yahoo Celebrity 4 ideas
- India Yahoo Finance 0 ideas
- India Yahoo Movies 16 ideas
- India Yahoo News 0 ideas
- India Yahoo Style 14 ideas
- Indonesia Celebrity 38 ideas
- Indonesia Homepage 1,165 ideas
- Indonesia News 170 ideas
- Indonesia Safely 29 ideas
- Indonesia She 34 ideas
- Ireland Homepage 90 ideas
- Jordan Maktoob Homepage 419 ideas
- Mail Ad Feedback 10 ideas
- Maktoob الطقس مكتوب 5 ideas
- Maktoob Celebrity 1 idea
- Maktoob Entertainment 10 ideas
- Maktoob Lifestyle 0 ideas
- Maktoob Movies 2 ideas
- Maktoob News 182 ideas
- Maktoob Screen 15 ideas
- Maktoob Style 1 idea
- Maktoob ألعاب مكتوب 0 ideas
- Maktoob شاشة مكتوب 28 ideas
- Malaysia Homepage 17 ideas
- Malaysia News 58 ideas
- Malaysia Safely 7 ideas
- Malaysia Video 0 ideas
- Malaysia Weather 1 idea
- Merchant Solutions 1 idea
- My Yahoo 31,967 ideas
- My Yahoo - back up 1 idea
- My Yahoo - US 9,176 ideas
- My Yahoo archive 314 ideas
- New Mail 11,359 ideas
- New Mail* 3,165 ideas
- New Zealand Business & Finance 132 ideas
- New Zealand Homepage 1,039 ideas
- New Zealand Safely 3 ideas
- New Zealand Screen 0 ideas
- PH ANC News 21 ideas
- Philippines Celebrity 214 ideas
- Philippines Homepage 9 ideas
- Philippines News 123 ideas
- Philippines Safely 12 ideas
- Philippines Video 0 ideas
- Philippines Weather 3 ideas
- Pick N Roll 19 ideas
- Postmaster 43 ideas
- Pro Football Pick'em 103 ideas
- Retail Pulse 0 ideas
- Rivals 11 ideas
- Safely 165 ideas
- Screen for iOS 0 ideas
- Search Extensions 98 ideas
- Search Product Downloads 89 ideas
- Security 497 ideas
- Sign-In Experience 79 ideas
- Singapore Entertainment 20 ideas
- Singapore Finance 230 ideas
- Singapore Homepage 1,052 ideas
- Singapore News 214 ideas
- Singapore Safely 11 ideas
- Singapore Screen 19 ideas
- Singapore Weather 4 ideas
- Singapore Yahoo Beauty 0 ideas
- Singapore Yahoo Celebrity 4 ideas
- Singapore Yahoo Finance 0 ideas
- Singapore Yahoo Movies 0 ideas
- Singapore Yahoo News 0 ideas
- Singapore Yahoo Style 4 ideas
- South Africa Celebrity 8 ideas
- South Africa Homepage 374 ideas
- South Africa News 23 ideas
- Sports Android 1,534 ideas
- Sports CA 35 ideas
- Sports iOS 1,026 ideas
- Sports Redesign 3,206 ideas
- SportsReel 6 ideas
- StatTracker Beta 581 ideas
- Survival Football 81 ideas
- Taiwan Yahoo 名人娛樂 0 ideas
- Taiwan Yahoo 運動 0 ideas
- Test 0 ideas
- Thailand Safely 2 ideas
- Toolbar Mail App 216 ideas
- Toolbar Weather App 72 ideas
- Tourney Pick'em 44 ideas
- UK & Ireland Finance 1,077 ideas
- UK & Ireland Games 19 ideas
- UK & Ireland Homepage 455 ideas
- UK & Ireland News 0 ideas
- UK & Ireland News Internal bucket 0 ideas
- UK & Ireland News Lego 378 ideas
- UK & Ireland Safely 38 ideas
- UK & Ireland TV 21 ideas
- UK & Ireland Video 187 ideas
- UK & Ireland Weather 100 ideas
- UK Answers 1 idea
- UK Daily Fantasy 1 idea
- UK Finance Mobile Android 12 ideas
- UK Finance Mobile DF iOS 2 ideas
- UK Finance Mobile iOS 310 ideas
- UK Yahoo Movies 23 ideas
- US Answers 8,999 ideas
- US Answers Mobile Web 2,156 ideas
- US Autos GS 442 ideas
- US Celebrity GS 661 ideas
- US Comments 350 ideas
- US Finance Mobile Android 44 ideas
- US Finance Mobile iOS 582 ideas
- US Flickr 267 ideas
- US Groups 4,225 ideas
- US Homepage B1 68 ideas
- US Homepage B2 33 ideas
- US Homepage B3 50 ideas
- US Homepage B4 33 ideas
- US Homepage B5 0 ideas
- US Homepage M 7,021 ideas
- US Homepage YDC 43 ideas
- US Homes GS 203 ideas
- US Live Web Insights 24 ideas
- US Mail 193 ideas
- US Mail 12,398 ideas
- US Maps 3,491 ideas
- US Membership Desktop 8,189 ideas
- US Membership Mobile 91 ideas
- US Movies GS 424 ideas
- US Music GS 195 ideas
- US News 6,057 ideas
- US Search App Android 2 ideas
- US Search App iOS 13 ideas
- US Search Chrome Extension 780 ideas
- US Search Chrome Extension v2 2,197 ideas
- US Search Desktop 1 idea
- US Search Desktop Bucket A 7 ideas
- US Search Desktop Bucket B 8 ideas
- US Search KG 14 ideas
- US Search Local Listings 20,797 ideas
- US Search Mobile Web 1 idea
- US Search Mozilla 0 ideas
- US Search Stock Quotes 11 ideas
- US Search Tablet Web 1 idea
- US Shine GS 1 idea
- US Toolbar 5,548 ideas
- US Travel GS 207 ideas
- US TV GS 367 ideas
- US Weather 2,322 ideas
- US Weather Bucket 0 ideas
- US Weather Mobile 13 ideas
- US Weather Mobile Android 2 ideas
- Video Guide Android 150 ideas
- Video Guide iOS 207 ideas
- Video Guide Testing 15 ideas
- Web Hosting 4 ideas
- Whitelist Yahoo Mail 0 ideas
- Yahoo Accessibility 359 ideas
- Yahoo Autos 71 ideas
- Yahoo Beauty 102 ideas
- Yahoo Celebrity 0 ideas
- Yahoo Celebrity Canada 0 ideas
- Yahoo Decor 0 ideas
- Yahoo Entertainment 357 ideas
- Yahoo Esports 50 ideas
- Yahoo Feedback 0 ideas
- Yahoo Finance Feedback Forum 1 idea
- Yahoo Finance IN Mobile Android 0 ideas
- Yahoo Finance SG Mobile Android 1 idea
- Yahoo FinanceReel 4 ideas
- Yahoo Food 118 ideas
- Yahoo Gemini 2 ideas
- Yahoo Health 90 ideas
- Yahoo Help 340 ideas
- Yahoo Home 240 ideas
- Yahoo Home* 28 ideas
- Yahoo Lifestyle 168 ideas
- Yahoo Live 0 ideas
- Yahoo Mail 2,348 ideas
- Yahoo Mail Android App 415 ideas
- Yahoo Mail Basic 643 ideas
- Yahoo Mail iOS App 55 ideas
- Yahoo Mail Mobile Web 1 idea
- Yahoo Makers 51 ideas
- Yahoo Messenger 93 ideas
- Yahoo Mobile Developer Suite 61 ideas
- Yahoo Mobile for Phone 15 ideas
- Yahoo Mobile for Tablet 0 ideas
- Yahoo Music 78 ideas
- Yahoo News Digest Android 870 ideas
- Yahoo News Digest iPad 0 ideas
- Yahoo News Digest iPhone 1,531 ideas
- Yahoo Newsroom Android App 59 ideas
- Yahoo Newsroom iOS App 34 ideas
- Yahoo Parenting 63 ideas
- Yahoo Politics 118 ideas
- Yahoo Publishing 13 ideas
- Yahoo Real Estate 2 ideas
- Yahoo Tech 461 ideas
- Yahoo Travel 143 ideas
- Yahoo TV 103 ideas
- Yahoo View 217 ideas
- Yahoo Weather Android 2,142 ideas
- Yahoo Weather iOS 22,810 ideas
- Yahoo! 7 Food App (iOS) 0 ideas
- Yahoo! 7 Homepage Archive 57 ideas
- Yahoo! 7 News (iOS) 23 ideas
- Yahoo! 7 Screen 0 ideas
- Yahoo! 7 TV FANGO App (Android) 1 idea
- Yahoo! 7 TV FANGO App (iOS) 1 idea
- Yahoo! 7 TV Guide App (Android) 0 ideas
- Yahoo! 7 TV Guide App (iOS) 1,249 ideas
- Yahoo! 7 TV Plus7 App (iOS) 0 ideas
- Yahoo! Concept Test Feedback Center 174 ideas
- Yahoo! Contributor Network 1 idea
- Yahoo! Transliteration 29 ideas
- YAHOO!7 Finance 553 ideas
- Yahoo!7 Games 9 ideas
- Yahoo!7 Safely 19 ideas
- Yahoo7 Finance Mobile DF iOS 12 ideas
- Yahoo7 Finance Mobile iOS 217 ideas
- Yahoo7 Homepage 2,549 ideas
Your password has been reset
We have made changes to increase our security and have reset your password.
We've just sent you an email to . Click the link to create a password, then come back here and sign in.
Atomic Structures: Pauli Exclusion Principle, Aufbau Principle & Hund's Rule
An error occurred trying to load this video.
Try refreshing the page, or contact customer support.
You must create an account to continue watching
Register for a free trial
As a member, you'll also get unlimited access to over 70,000 lessons in math, English, science, history, and more. Plus, get practice tests, quizzes, and personalized coaching to help you succeed.
Already registered? Login here for access
You're on a roll. Keep up the good work!
Just checking in. Are you still watching?
- 0:00 Review of Quantum Numbers
- 3:15 Pauli Exclusion Principle
- 4:22 Hund's Rule
- 4:46 Aufbau Principle
- 6:30 Lesson Summary
Want to watch this again later?
Log in or sign up to add this lesson to a Custom Course.
Organize and save your favorite lessons with Custom Courses
Recommended Lessons and Courses for You








































Amy has taught university-level earth science courses and has a PhD in Geology.
Review of Quantum Numbers
At this point in studying chemistry, when you visualize an atom, you likely think of a nice orderly structure with a nucleus of positively charged protons and neutral neutrons that are orbited by rings of electrons, much like the structure of the solar system with planets orbiting the sun. This model of an atom follows the Bohr model, which has a positively charged nucleus of protons and neutrons surrounded by fixed rings of electrons called shells.
We can think of atomic structure like a hotel. Just like there are a fixed number of rooms where guests may stay, there are fixed locations where we are likely to find electrons. These fixed locations are called orbitals and their location is defined by their quantum number. Quantum numbers allow us to define the location of an electron within the atomic structure. Much like a hotel room, the orbitals are located at different floors, or levels, of the structure, and can even be described by the shape of the wing in which they are located.
In this model, the shells correspond to energy levels in the atomic structure, with the shells closer to the nucleus having the lowest energy level. This energy level is also defined as the first quantum number, n. So, an electron in the first shell would have a first quantum number (or n) of one. If it was in the second shell, it would have a quantum number of two, and so on. If you think of our hotel analogy, the levels towards the bottom take the least amount of energy to get to, and thus these levels have the lowest energy.
Within each shell, we find subshells that have different shapes depending on their energy level and thus can fit a different number of electrons. They are named after the letters s, p, d, f and so on. These subshells are the second quantum number and are numerically defined as follow:
letter = level (l)
The first shell has just the s subshell. The second has s and p. The third has s, p, and d. The higher the first quantum number or the shell, the more subshells and thus electrons can fit into that structure.
The third quantum number, m sub l, specifies the orientation of a specific orbital at a given n and s. This quantum number allows us to break up each subshell into individual orbitals of two electrons each. We can calculate the number of orbitals by the equation 2l + 1. Thus, according to this equation, the s shell would have 1 orbital with 2 electrons. The p shell would have 3 orbitals with 6 electrons. The d shell would have 5 orbitals with 10 electrons. The f would have 7 orbitals with 14 electrons.
Pauli Exclusion Principle
So, according to these rules, if we look at the first shell, it only contains two electrons in the first subshell. The quantum number can be described as 1s^2. However, another rule is that two electrons cannot have identical quantum numbers. So, if two electrons have to fit inside one s subshell, how will this work?
This matter directly relates to the Pauli Exclusion Principle, which states that two electrons cannot exist in the same location and thus electrons in the same orbital must have opposing spins. Hydrogen only has one electron in the 1s orbital and has a quantum number of 1s^1. Helium has two electrons in this field, so they must have opposing spins, and it has a quantum number of 1s^2.
This spin factor is the fourth quantum number, m, and is described as either +1/2 for a spin 'up' or -1/2 for a spin 'down.'
Hund's Rule
So now that we've reviewed the orbital structure, let's dive in to how the electrons fill these orbitals. Because electrons are both negatively charged, there is a certain amount of repulsion that prevents them from wanting to fill the same space. So, according to Hund's Rule, electrons will fall into empty orbitals of the same energy before electrons begin to pair up into the same orbital.
Aufbau Principle
Closely related to Hund's Rule is the Aufbau Principle, which states that electrons will fill the lower energy levels before moving to higher energy orbitals. Remember that each orbital has two electrons and the number of orbitals at an energy level depends on the first two quantum numbers.
Unlock Content
Get FREE access for 5 days,
just create an account.
No obligation, cancel anytime.
Select a subject to preview related courses:
Let's combine Hund's Rule and the Aufbau Principle by looking at the electron configuration for two very common elements. Nitrogen has an atomic number of 7 and thus has 7 protons and electrons. Next to it on the periodic table is oxygen with an atomic number of 8 and thus 8 protons and electrons. The s subshells are lower energy in the first and second shells and thus fill first. The p subshell is filled with one electron each in nitrogen; however, since oxygen has four electrons that go into this subshell, only when the three orbitals already have one electron does it completely fill an orbital.
In general, the energy levels of the orbitals increase with the first and second quantum numbers, which are the shell and the subshell. However, as you start getting to higher energies, this relationship gets more complicated because the s subshells of higher levels may have lower energy than the d and f subshells of lower levels. The lines on this diagram show the direction of increasing energy:

You will notice that the 4s subshell has lower energy than the 3d subshell. You do not have to memorize these relationships, but just remember that when you start getting to the higher energy levels, consult this chart to help you determine the quantum number.
Lesson Summary
In summary, Hund's Rule, the Aufbau Principle, and the Pauli Exclusion Principle help us define how electrons fill the orbitals within an atomic structure. This helps us predict the location of electrons in an atom as we increase the number of electrons. The Pauli Exclusion Principle states that no two electrons can have the same quantum number, and thus, electrons in the same orbital must have opposite spins. Remember the quantum number is the numerical description of the likely location of the electron, so you can think of it as the electron's address.
The Aufbau Principle states that electrons fill orbitals of lower energy first before moving to higher energy orbitals. So you must fill all of the orbital spaces in the 2s orbital before moving on to the 2p orbitals.
Finally, Hund's Rule says that because of the repulsion between the two negatively charged electrons, a newly added electron will go into an empty orbital of the same energy before going into an orbital that already has an electron present. These rules help us predict how the orbital spaces will be filled by elements on the periodic table as we move to larger elements with more electrons.
Learning Outcomes
Following this lesson, you'll have the ability to:
- Define electron shells, orbitals and quantum numbers
- Recall what the Bohr model is
- Describe the Pauli Exclusion Principle, Hund's Rule and the Aufbau Principle
- Explain how the three concepts above can be used to predict how orbital spaces will be filled with electrons
To unlock this lesson you must be a Study.com Member.
Register for a free trial
Unlock Your Education
See for yourself why 30 million people use Study.com
Become a Study.com member and start learning now.
Already a member? Log In
Earning College Credit
Did you know… We have over 95 college courses that prepare you to earn credit by exam that is accepted by over 2,000 colleges and universities. You can test out of the first two years of college and save thousands off your degree. Anyone can earn credit-by-exam regardless of age or education level.
Transferring credit to the school of your choice
Not sure what college you want to attend yet? Study.com has thousands of articles about every imaginable degree, area of study and career path that can help you find the school that's right for you.
Research Schools, Degrees & Careers
Get the unbiased info you need to find the right school.
Browse Articles By Category
Browse an area of study or degree level.
Recommended Articles
Chemistry: High School
19 chapters | 179 lessons | 1 flashcard set
- Go to Measurement and Problem Solving
- Go to What Is Matter?
- The Atom 4:09
- Atomic Number and Mass Number 9:15
- Early Atomic Theory: Dalton, Thomson, Rutherford and Millikan 6:35
- Isotopes and Average Atomic Mass 7:29
- Avogadro's Number: Using the Mole to Count Atoms 9:15
- Electron Configurations in Atomic Energy Levels 10:40
- Atomic Structures: Pauli Exclusion Principle, Aufbau Principle & Hund's Rule 7:54
- 5:03

- Go to The Representative Elements of the Periodic Table
- Go to Bonding for High School Chemistry
- Go to Gases in Chemistry
- Go to Stoichiometry and Chemical Equations
- Go to Equilibrium
- Go to Thermodynamics in Chemistry
- Go to Teaching Resources for High School Chemistry
Atomic Structures: Pauli Exclusion Principle, Aufbau Principle & Hund's Rule Related Study Materials
Browse by Courses
Browse by Lessons
Latest Courses
Latest Lessons
Popular Courses
Popular Lessons
Explore our library of over 70,000 lessons
Download the app

About Us
Download the app

© copyright 2003-2018 Study.com. All other trademarks and copyrights are the property of their respective owners. All rights reserved.
Create your account. No obligation; cancel anytime.
Start your FREE trial. No obligation; cancel anytime.
Your selected plan:
You are joining:
Your Cart is Empty. Please Choose a Product.
Study.com video lessons have helped over 30 million students.
Students Love Study.com
"I learned more in 10 minutes than 1 month of chemistry classes"
Earn College Credit
"I aced the CLEP exam and earned 3 college credits!"
Study.com video lessons have helped over half a million teachers engage their students.
Teachers Love Study.com
"The videos have changed the way I teach! The videos on Study.com accomplish in 5 minutes what would take me an entire class."
Did you know.
Students in online learning conditions performed better than those receiving face-to-face instruction.
Hund s
- Interface:
- English
- Français
- Español
- Deutsch
- Italiano
- Português
- Reverso Context Mobile app
- Conditions of use
- Copyright
der Große/Kleine Hund (Astron) Great(er)/Little or Lesser Dog
junger Hund puppy, pup
die Familie der Hunde the dog or canine family
Hunde, die (viel) bellen, beißen nicht (Prov) empty vessels make most noise (Prov)
getroffene Hunde bellen inf if the cap fits, wear it
viele Hunde sind des Hasen Tod (Prov) there is not much one person can do against many
wie Hund und Katze leben to live like cat and dog, to lead a cat-and-dog life
ich würde bei diesem Wetter keinen Hund auf die Straße jagen I wouldn't send a dog out in this weather
damit kann man keinen Hund hinterm Ofen hervorlocken inf that's not going to tempt anybody
müde wie ein Hund sein inf to be dog-tired
er ist bekannt wie ein bunter Hund inf everybody knows him
kein Hund nimmt ein Stück Brot von ihm everyone avoids him like the plague
das ist (ja) zum Junge-Hunde-Kriegen inf it's enough to give you kittens
da wird der Hund in der Pfanne verrückt inf it's enough to drive you crazy inf or round the twist (Brit) inf
da liegt der Hund begraben inf (so) that's what is/was behind it all , (Haken, Problem etc) that's the problem
er ist mit allen Hunden gehetzt inf he knows all the tricks, there are no flies on him (Brit) inf
er ist ein armer Hund he's a poor soul or devil inf
er ist völlig auf dem Hund inf he's really gone to the dogs inf
auf den Hund kommen inf to go to the dogs inf
jdn auf den Hund bringen inf to ruin sb , (gesundheitlich) to ruin sb's health
die Weiber haben/der Suff hat ihn auf den Hund gebracht inf women have/drink has been his ruin or downfall
vor die Hunde gehen inf to go to the dogs inf (=sterben) to die, to perish (=getötet werden) to cop it (Brit) inf , to be killed
du blöder Hund inf you silly or stupid bastard sl
du gemeiner Hund inf you rotten bastard sl
du schlauer or gerissener Hund inf you sly or crafty devil or old fox
schlafende Hunde soll man nicht wecken (prov) let sleeping dogs lie (Prov)
Results found in: English-German
- you rat! exp. du Hund!
- sly dog exp. gerissener Hund
- dirty dog exp. gemeiner Hund
- to give a dog a scratch exp. einen Hund kratzen
- a poor blighter exp. ein armer Hund
Examples and translations in context
Alphabetical index
Welcome to German-English Collins dictionary. Type the word that you look for in the search box above. The results will include words and phrases from the general dictionary as well as entries from the collaborative one.
US Search Desktop

We appreciate your feedback on how to improve Yahoo Search. This forum is for you to make product suggestions and provide thoughtful feedback. We’re always trying to improve our products and we can use the most popular feedback to make a positive change!
If you need assistance of any kind, please visit our community support forum or find self-paced help on our help site. This forum is not monitored for any support-related issues.
The Yahoo product feedback forum now requires a valid Yahoo ID and password to participate.
You are now required to sign-in using your Yahoo email account in order to provide us with feedback and to submit votes and comments to existing ideas. If you do not have a Yahoo ID or the password to your Yahoo ID, please sign-up for a new account.
If you have a valid Yahoo ID and password, follow these steps if you would like to remove your posts, comments, votes, and/or profile from the Yahoo product feedback forum.
- Vote for an existing idea ( )
- or
- Post a new idea…
- Hot ideas
- Top ideas
- New ideas
- Category
- Status
- My feedback
Put on the computer what the public asks for, not everything about the US.
I asked for a total medal count. I have been looking for 20 minutes and still cannot find it.
I also asked for a specific medal count for a specific country and got a history of when they first started to compete in the Olympics. I asked for a medal count for 2018, as of today,
not a history of that country.
You don't even accept what I have asked.
- Don't see your idea?
- Post a new idea…
US Search Desktop
- Post a new idea…
- All ideas
- My feedback
- I have a problem 24
- I have a suggestion 20
- Other 3
- What I dislike 29
Feedback and Knowledge Base
Give feedback
- Deutschland Finanzen Mobile DF iOS 1 idea
- España Finanzas Mobile DF iOS 7 ideas
- Accounts Dashboard 33 ideas
- Ad feedback 3 ideas
- Answers TH 31 ideas
- Answers TH 0 ideas
- Answers UV Forum (test version) 10 ideas
- Australia Celebrity 0 ideas
- Australia Finance Mobile Android 0 ideas
- Australia Style 0 ideas
- Australia Yahoo Tech 0 ideas
- Autos Pulse 2 ideas
- Aviate 1,513 ideas
- Canada Finance 1,099 ideas
- Canada Finance Mobile Android 0 ideas
- Canada Finance Mobile DF iOS 3 ideas
- Canada Finance Mobile iOS 469 ideas
- Canada Homepage 5,130 ideas
- Canada Movies 14 ideas
- Canada News 873 ideas
- Canada Safely 10 ideas
- Canada Screen 128 ideas
- Canada Weather 94 ideas
- Canada Yahoo Beauty 0 ideas
- Canada Yahoo Celebrity 10 ideas
- Canada Yahoo Finance 0 ideas
- Canada Yahoo Movies 10 ideas
- Canada Yahoo News 0 ideas
- Canada Yahoo Style 21 ideas
- College Football Pick'em 112 ideas
- Connected TV 362 ideas
- Corp Mail Test 1 1,313 ideas
- Corp Mail Testing 1,256 ideas
- Cricket 24 ideas
- Daily Fantasy 89 ideas
- Developer Network 1 idea
- Double Down 86 ideas
- Fantasy Baseball 455 ideas
- Fantasy Basketball 402 ideas
- Fantasy Football 707 ideas
- Fantasy Hockey 354 ideas
- Fantasy Live Scoring on Matchup and Standings 811 ideas
- Fantasy Ratings and Levels 7 ideas
- Fantasy Sports Android Apps 1,367 ideas
- Fantasy Sports iOS Apps 2,127 ideas
- Finance 1,248 ideas
- Finance - CA 495 ideas
- Finance - US 9 ideas
- Finance ChartIQ 443 ideas
- Finance Mobile Web 403 ideas
- Finance Portfolios 810 ideas
- Finance Stock Screener 35 ideas
- Finance Tablet 44 ideas
- Flickr - Profile 290 ideas
- Flickr Android 60 ideas
- Flickr for Apple TV 27 ideas
- Flickr Groups 13 ideas
- Flickr Internal 0 ideas
- Flickr iOS Dogfooding 0 ideas
- Flickr iPad 152 ideas
- Flickr iPhone 370 ideas
- Flickr New Photo Page 8,030 ideas
- Flickr Search 0 ideas
- Food Magazines 0 ideas
- Games 3,147 ideas
- Global Maps 1,024 ideas
- GS Mobile Web 42 ideas
- Health Pulse 3 ideas
- Home Page (Android) 1,689 ideas
- Home Page (iOS) 3,809 ideas
- Hong Kong Homepage 0 ideas
- India Celebrity 43 ideas
- India Finance 493 ideas
- India Homepage 1,872 ideas
- India Lifestyle 173 ideas
- India Movies 84 ideas
- India News 334 ideas
- India Partner Portal Tata 0 ideas
- India Partner Portal Tikona 0 ideas
- India Safely 15 ideas
- India Screen 165 ideas
- India Weather 30 ideas
- India Yahoo Beauty 0 ideas
- India Yahoo Celebrity 4 ideas
- India Yahoo Finance 0 ideas
- India Yahoo Movies 16 ideas
- India Yahoo News 0 ideas
- India Yahoo Style 14 ideas
- Indonesia Celebrity 38 ideas
- Indonesia Homepage 1,165 ideas
- Indonesia News 170 ideas
- Indonesia Safely 29 ideas
- Indonesia She 34 ideas
- Ireland Homepage 90 ideas
- Jordan Maktoob Homepage 419 ideas
- Mail Ad Feedback 10 ideas
- Maktoob الطقس مكتوب 5 ideas
- Maktoob Celebrity 1 idea
- Maktoob Entertainment 10 ideas
- Maktoob Lifestyle 0 ideas
- Maktoob Movies 2 ideas
- Maktoob News 182 ideas
- Maktoob Screen 15 ideas
- Maktoob Style 1 idea
- Maktoob ألعاب مكتوب 0 ideas
- Maktoob شاشة مكتوب 28 ideas
- Malaysia Homepage 17 ideas
- Malaysia News 58 ideas
- Malaysia Safely 7 ideas
- Malaysia Video 0 ideas
- Malaysia Weather 1 idea
- Merchant Solutions 1 idea
- My Yahoo 31,967 ideas
- My Yahoo - back up 1 idea
- My Yahoo - US 9,176 ideas
- My Yahoo archive 314 ideas
- New Mail 11,359 ideas
- New Mail* 3,165 ideas
- New Zealand Business & Finance 132 ideas
- New Zealand Homepage 1,039 ideas
- New Zealand Safely 3 ideas
- New Zealand Screen 0 ideas
- PH ANC News 21 ideas
- Philippines Celebrity 214 ideas
- Philippines Homepage 9 ideas
- Philippines News 123 ideas
- Philippines Safely 12 ideas
- Philippines Video 0 ideas
- Philippines Weather 3 ideas
- Pick N Roll 19 ideas
- Postmaster 43 ideas
- Pro Football Pick'em 103 ideas
- Retail Pulse 0 ideas
- Rivals 11 ideas
- Safely 165 ideas
- Screen for iOS 0 ideas
- Search Extensions 98 ideas
- Search Product Downloads 89 ideas
- Security 497 ideas
- Sign-In Experience 79 ideas
- Singapore Entertainment 20 ideas
- Singapore Finance 230 ideas
- Singapore Homepage 1,052 ideas
- Singapore News 214 ideas
- Singapore Safely 11 ideas
- Singapore Screen 19 ideas
- Singapore Weather 4 ideas
- Singapore Yahoo Beauty 0 ideas
- Singapore Yahoo Celebrity 4 ideas
- Singapore Yahoo Finance 0 ideas
- Singapore Yahoo Movies 0 ideas
- Singapore Yahoo News 0 ideas
- Singapore Yahoo Style 4 ideas
- South Africa Celebrity 8 ideas
- South Africa Homepage 374 ideas
- South Africa News 23 ideas
- Sports Android 1,534 ideas
- Sports CA 35 ideas
- Sports iOS 1,026 ideas
- Sports Redesign 3,206 ideas
- SportsReel 6 ideas
- StatTracker Beta 581 ideas
- Survival Football 81 ideas
- Taiwan Yahoo 名人娛樂 0 ideas
- Taiwan Yahoo 運動 0 ideas
- Test 0 ideas
- Thailand Safely 2 ideas
- Toolbar Mail App 216 ideas
- Toolbar Weather App 72 ideas
- Tourney Pick'em 44 ideas
- UK & Ireland Finance 1,077 ideas
- UK & Ireland Games 19 ideas
- UK & Ireland Homepage 455 ideas
- UK & Ireland News 0 ideas
- UK & Ireland News Internal bucket 0 ideas
- UK & Ireland News Lego 378 ideas
- UK & Ireland Safely 38 ideas
- UK & Ireland TV 21 ideas
- UK & Ireland Video 187 ideas
- UK & Ireland Weather 100 ideas
- UK Answers 1 idea
- UK Daily Fantasy 1 idea
- UK Finance Mobile Android 12 ideas
- UK Finance Mobile DF iOS 2 ideas
- UK Finance Mobile iOS 310 ideas
- UK Yahoo Movies 23 ideas
- US Answers 8,999 ideas
- US Answers Mobile Web 2,156 ideas
- US Autos GS 442 ideas
- US Celebrity GS 661 ideas
- US Comments 350 ideas
- US Finance Mobile Android 44 ideas
- US Finance Mobile iOS 582 ideas
- US Flickr 267 ideas
- US Groups 4,225 ideas
- US Homepage B1 68 ideas
- US Homepage B2 33 ideas
- US Homepage B3 50 ideas
- US Homepage B4 33 ideas
- US Homepage B5 0 ideas
- US Homepage M 7,021 ideas
- US Homepage YDC 43 ideas
- US Homes GS 203 ideas
- US Live Web Insights 24 ideas
- US Mail 193 ideas
- US Mail 12,398 ideas
- US Maps 3,491 ideas
- US Membership Desktop 8,189 ideas
- US Membership Mobile 91 ideas
- US Movies GS 424 ideas
- US Music GS 195 ideas
- US News 6,057 ideas
- US Search App Android 2 ideas
- US Search App iOS 13 ideas
- US Search Chrome Extension 780 ideas
- US Search Chrome Extension v2 2,197 ideas
- US Search Desktop 1 idea
- US Search Desktop Bucket A 7 ideas
- US Search Desktop Bucket B 8 ideas
- US Search KG 14 ideas
- US Search Local Listings 20,797 ideas
- US Search Mobile Web 1 idea
- US Search Mozilla 0 ideas
- US Search Stock Quotes 11 ideas
- US Search Tablet Web 1 idea
- US Shine GS 1 idea
- US Toolbar 5,548 ideas
- US Travel GS 207 ideas
- US TV GS 367 ideas
- US Weather 2,322 ideas
- US Weather Bucket 0 ideas
- US Weather Mobile 13 ideas
- US Weather Mobile Android 2 ideas
- Video Guide Android 150 ideas
- Video Guide iOS 207 ideas
- Video Guide Testing 15 ideas
- Web Hosting 4 ideas
- Whitelist Yahoo Mail 0 ideas
- Yahoo Accessibility 359 ideas
- Yahoo Autos 71 ideas
- Yahoo Beauty 102 ideas
- Yahoo Celebrity 0 ideas
- Yahoo Celebrity Canada 0 ideas
- Yahoo Decor 0 ideas
- Yahoo Entertainment 357 ideas
- Yahoo Esports 50 ideas
- Yahoo Feedback 0 ideas
- Yahoo Finance Feedback Forum 1 idea
- Yahoo Finance IN Mobile Android 0 ideas
- Yahoo Finance SG Mobile Android 1 idea
- Yahoo FinanceReel 4 ideas
- Yahoo Food 118 ideas
- Yahoo Gemini 2 ideas
- Yahoo Health 90 ideas
- Yahoo Help 340 ideas
- Yahoo Home 240 ideas
- Yahoo Home* 28 ideas
- Yahoo Lifestyle 168 ideas
- Yahoo Live 0 ideas
- Yahoo Mail 2,348 ideas
- Yahoo Mail Android App 415 ideas
- Yahoo Mail Basic 643 ideas
- Yahoo Mail iOS App 55 ideas
- Yahoo Mail Mobile Web 1 idea
- Yahoo Makers 51 ideas
- Yahoo Messenger 93 ideas
- Yahoo Mobile Developer Suite 61 ideas
- Yahoo Mobile for Phone 15 ideas
- Yahoo Mobile for Tablet 0 ideas
- Yahoo Music 78 ideas
- Yahoo News Digest Android 870 ideas
- Yahoo News Digest iPad 0 ideas
- Yahoo News Digest iPhone 1,531 ideas
- Yahoo Newsroom Android App 59 ideas
- Yahoo Newsroom iOS App 34 ideas
- Yahoo Parenting 63 ideas
- Yahoo Politics 118 ideas
- Yahoo Publishing 13 ideas
- Yahoo Real Estate 2 ideas
- Yahoo Tech 461 ideas
- Yahoo Travel 143 ideas
- Yahoo TV 103 ideas
- Yahoo View 217 ideas
- Yahoo Weather Android 2,142 ideas
- Yahoo Weather iOS 22,810 ideas
- Yahoo! 7 Food App (iOS) 0 ideas
- Yahoo! 7 Homepage Archive 57 ideas
- Yahoo! 7 News (iOS) 23 ideas
- Yahoo! 7 Screen 0 ideas
- Yahoo! 7 TV FANGO App (Android) 1 idea
- Yahoo! 7 TV FANGO App (iOS) 1 idea
- Yahoo! 7 TV Guide App (Android) 0 ideas
- Yahoo! 7 TV Guide App (iOS) 1,249 ideas
- Yahoo! 7 TV Plus7 App (iOS) 0 ideas
- Yahoo! Concept Test Feedback Center 174 ideas
- Yahoo! Contributor Network 1 idea
- Yahoo! Transliteration 29 ideas
- YAHOO!7 Finance 553 ideas
- Yahoo!7 Games 9 ideas
- Yahoo!7 Safely 19 ideas
- Yahoo7 Finance Mobile DF iOS 12 ideas
- Yahoo7 Finance Mobile iOS 217 ideas
- Yahoo7 Homepage 2,549 ideas
Your password has been reset
We have made changes to increase our security and have reset your password.
We've just sent you an email to . Click the link to create a password, then come back here and sign in.
Комментариев нет:
Отправить комментарий